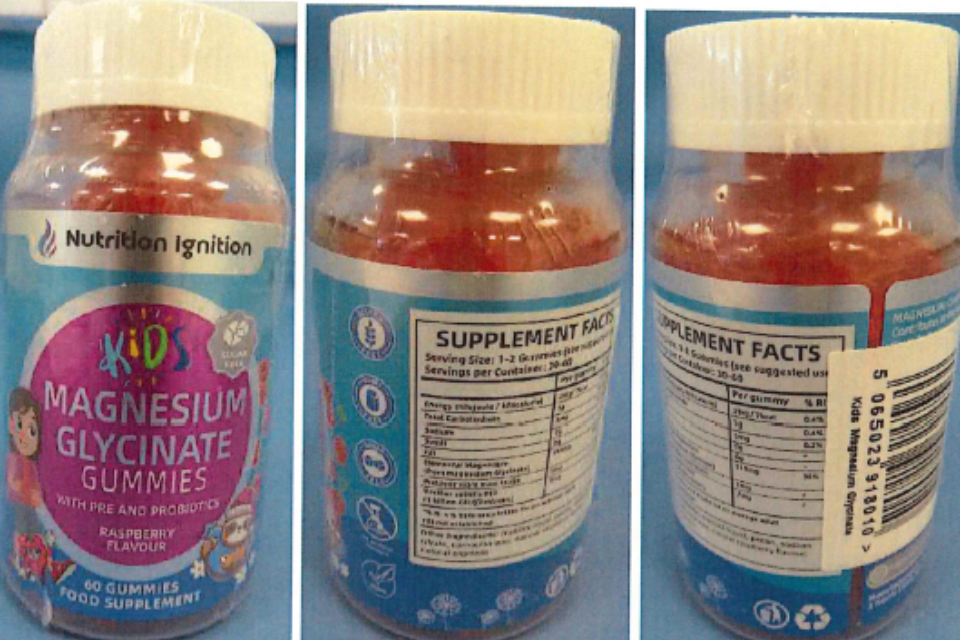
The MHRA’s Warning on Nutrition Ignition Kids Magnesium Glycinate Gummies: A Call to Action for Parents
In households across the UK, parents often turn to dietary supplements to support their children’s health and well-being. However, a recent alert from the Medicines and Healthcare products Regulatory Agency (MHRA) has sent shockwaves through the community, revealing the unexpected dangers lurking in seemingly innocuous gummy vitamins. The MHRA has advised parents to cease all use of Nutrition Ignition Kids Magnesium Glycinate Gummies due to the alarming discovery of undeclared melatonin, a substance typically reserved for prescription use in sleep disorders.
According to tests conducted by the MHRA, two batches of the gummies were found to contain 1.5 to 1.7mg of melatonin in each gummy, a dosage not indicated anywhere on the product’s packaging. This discrepancy raises significant concerns, especially considering that the gummies are marketed to children aged four and above for benefits such as “calm, focus, and digestion.” Dr. Alison Cave, MHRA Chief Safety Officer, emphasized the urgency of the situation: “We advise any parent or caregiver to stop use of this product and safely dispose of it.”
The Health Implications of Undeclared Melatonin
Melatonin is a hormone that regulates sleep-wake cycles and is generally safe when used appropriately. However, the unregulated presence of this hormone in a product aimed at children presents inherent risks. Overconsumption can lead to side effects like drowsiness, headaches, dizziness, and nausea, particularly concerning for younger children who may not clearly communicate their discomfort.
A study from the Institute of Pediatric Health found that while lasting harm from melatonin at high levels is unlikely and the body usually clears it within 12 hours, symptoms like hyperactivity and abdominal pain have been reported in children under prescribed conditions. Dr. Maria Johnston, a pediatric endocrinologist involved in the study, cautioned, “Even though melatonin’s safety profile is generally robust, the risk of side effects increases significantly in unregulated environments. Parents should remain vigilant.”
- Recommended usage: Typically 1mg for children, increasing to a maximum of 5mg per day.
- Common side effects: Drowsiness, headaches, dizziness, abdominal pain.
- Potential risk: Hyperactivity and other adverse reactions when overdosing occurs.
The Regulatory Landscape
The MHRA’s scrutiny highlights the complexities of classifying dietary supplements in relation to pharmaceutical products. The detection of melatonin within Nutrition Ignition’s gummies immediately necessitated reclassification; a product once marketed as a food supplement must now follow guidelines applicable to medicines. As stated by regulatory expert Professor Edward Harris, “This case illustrates the critical need for strict oversight in the supplement industry. We cannot afford to compromise the safety of our children.”
The MHRA, alongside online retailers, has swiftly moved to remove the offending product from sale, underscoring the seriousness of undeclared ingredients. Parents with the gummies at home are advised to secure them in a tamper-proof container, out of children’s reach, until proper disposal can be arranged at local pharmacies. This advice is more than just a precaution; it reflects a growing need for public awareness regarding the implications of unregulated supplements.
Public Reaction and Responsibility
Public responses have ranged from outrage to confusion, as many parents wonder how such a serious oversight could occur. Some have called for stricter regulations, while others urge manufacturers to enhance labeling transparency. The incident has spurred discussions in online parenting communities, with many sharing their apprehensions about dietary supplements traditionally viewed as harmless.
Lucia Bennett, a parent whose child used the gummies, expressed her disbelief: “I bought these thinking they would help promote calmness and better focus for my son during the school year. Now, I feel betrayed. It’s terrifying to think that I was feeding him something that could have hidden dangers.”
Experts urge a united approach to address such oversights. Dr. Cave noted, “While we have established mechanisms to ensure product safety, incidents like this remind us that ongoing vigilance is paramount. We urge parents and caregivers to report suspected side effects through the MHRA Yellow Card scheme, as community feedback is invaluable for improving safety.”
A Call for Increased Vigilance
The question remains: how can parents ensure the safety of the products they give their children? It’s a challenge in an increasingly complex market where the lines between dietary supplements and medicines can blur. Dr. Abigail Chen, a consumer safety advocate, suggests: “Educating oneself about the ingredients in products and their potential effects is crucial. Parents should look for published research and consult with healthcare professionals before introducing any new supplement into their child’s routine.”
As the dust settles from this unsettling episode, it becomes clear that the responsibility lies not only with the regulatory bodies and manufacturers but also with consumers themselves. Parents must remain proactive and informed, ensuring their children’s health and safety in this intricate landscape of dietary products.
This incident serves as a pivotal reminder: while the allure of gummies as a simple solution to a child’s nutrition may be enticing, due diligence in selecting and using dietary supplements is an imperative responsibility for caregivers.
Source: www.gov.uk